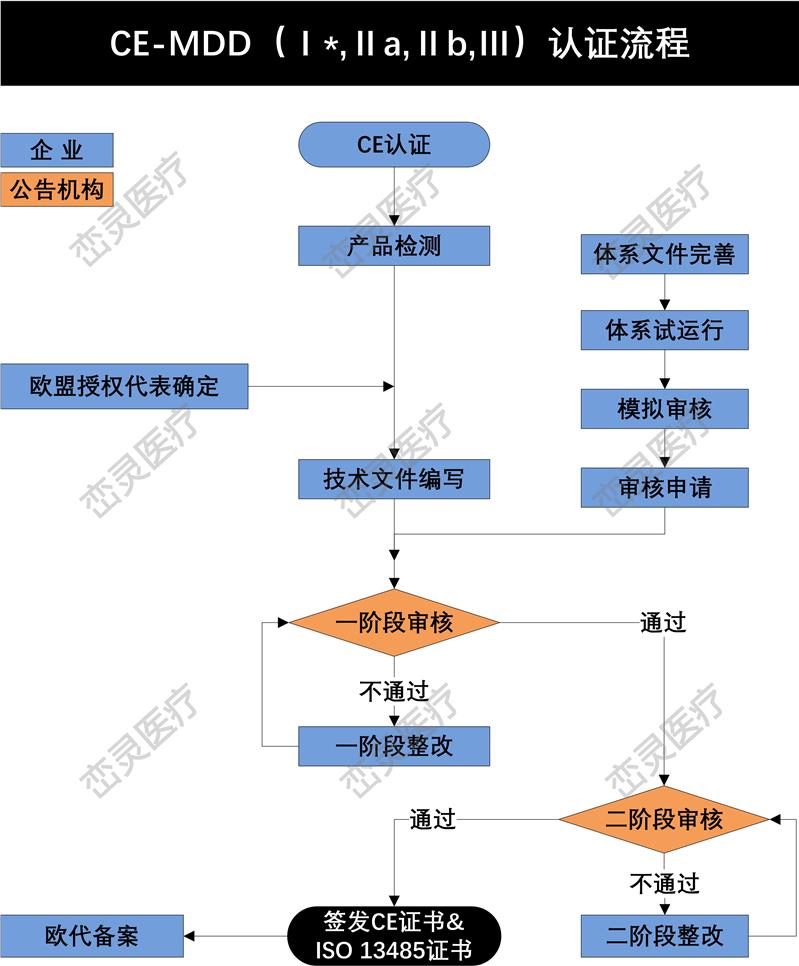
- 产品描述
上海峦灵将为您提供如下欧盟授权代表服务
产品于欧盟注册
非欧盟国家的制造商或出口商,其产品允许在欧盟市场销售前,应委任欧盟授权代表。只有当产品满足欧盟相关法规或指令要求,并且在产品或包装上标明授权代表的名称和,才允许在欧盟自由销售或使用。
Minimum requirements
Verify the Declaration of Conformity
Review the Technical Documentation
Check where applicable that an appropriate conformity assessment exist
Keep the previous documentation (Declaration of conformity, Technical Documentation, certificates issued by Notified Body and amendments) at
the disposal of competent authorities (at least 10 years and for implantable devices it's 15 years)
Verify that the manufacturer is complying with the registration of the
Unique Device Identification according to article 27
Minimum requirements:
Make sure that the registration of the device is performed according to
article 29
Also that the registration of the manufacturer, Authorized Representative and importers are done according to article 31
In case requested by the competent authorities, the Authorized Representative should give all the information and documentation necessary to prove the conformity of a device, in an official language
Keep the manufacturer informed of any request coming from the
competent authorities.
Verify that the competent authorities receives the samples or is given access to the device
? Located outside EU
Companies outside EU can be out of EU law as they have no place of business there.
? Want to sell Medical Devices To receive authorization to sell your Medical Devices in EU you have to
appoint one. It will be mandatory to get your CE certification from the Notified Body.
关于我们:峦灵是行业内**的咨询公司,汇聚了来自法规、等各领域对法规有深刻理解的人士及*,致力为企业提供一站式、*、系统化的技术咨询服务。迄今为止,我们已与众多公司开展合作, 协助他们将安全、有效、合规的产品迅速推向**市场。
峦灵是行业内的咨询公司,汇聚了来自法规、等各领域对法规有深刻理解的人士及*,致力为企业提供一站式、*、系统化的技术咨询服务, 帮助企业向**市场提供安全、有效和合规的产品, 迄今为止,我们已与众多公司开展合作, 协助他们将安全、有效、合规的产品迅速推向**市场。 我们的服务包括: 法规合规服务(CE认证、NMPA注册、FDA注册、**注册) 质量管理体系(ISO13485、NMPA GMP、MDSAP、FDA QSR820、质量体系日常维护服务); 测试服务(有源产品测试认证咨询、无源产品测试认证咨询、清洗消毒灭菌验证确认、定制化摸底测试服务) **注册(加拿大、澳大利亚、日本、巴西、韩国、俄罗斯、东南亚注等); 法规培训服务(企业定制培训、小班课培训)
欢迎来到上海峦灵医疗信息技术有限公司网站,我公司位于历史文化悠久,近代城市文化底蕴深厚,历史古迹众多,有“东方巴黎”美称的上海市。 具体地址是上海青浦公司街道地址,负责人是阮贞。
主要经营CE认证 FDA510K。
你有什么需要?我们都可以帮你一一解决!我们公司主要的特色服务是:CE认证,FDA注册,**注册等,“诚信”是我们立足之本,“创新”是我们生存之源,“便捷”是我们努力的方向,用户的满意是我们最大的收益、用户的信赖是我们最大的成果。
本页链接:http://www.cg160.cn/vgy-54364749.html
以上信息由企业自行发布,该企业负责信息内容的完整性、真实性、准确性和合法性。阿德采购网对此不承担任何责任。 马上查看收录情况: 百度 360搜索 搜狗
关于上海峦灵医疗信息技术有限公司
商铺首页 |
更多产品 |
联系方式
峦灵是行业内的咨询公司,汇聚了来自法规、等各领域对法规有深刻理解的人士及*,致力为企业提供一站式、*、系统化的技术咨询服务, 帮助企业向**市场提供安全、有效和合规的产品, 迄今为止,我们已与众多公司开展合作, 协助他们将安全、有效、合规的产品迅速推向**市场。 我们的服务包括: 法规合规服务(CE认证、NMPA注..
- 我要给“大同CE认证”留言
- 更多产品
相关分类